Brain-on-chip
MiSTR – Microfluidic-controlled stem cell regionalization
Human brain development is guided by concentration gradients of signalling cues. Along the rostro-caudal axis, Wingless-type proteins (WNTs) are responsible for defining the three early brain regions: forebrain, midbrain and hindbrain [1]. Other signalling cues, such as Sonic Hedgehogprotein (SHH) and Bone Morphogenetic proteins (BMPs), are secreted in gradients along the dorso-ventral axis (running perpendicular to the rostro-caudal axis) [2]. This yields more subtypes of neural progenitor cells within each brain region.
In vitro models such as brain organoids, have been used to model dorso-ventral patterning [3][4]. However, no model that accounts for the patterning of the rostro-caudal axis has been presented. To model the regionalization along the rostro-caudal axis, we developed the MiSTR model (Microfluidic-controlled stem cell regionalization), where human embryonic stem cells (hESCs) are exposed to a linear concentration gradient of a GSK3i inhibitor which, similar to WNTs, patterns the cells into neural progenitor cells of forebrain, midbrain and hindbrain.
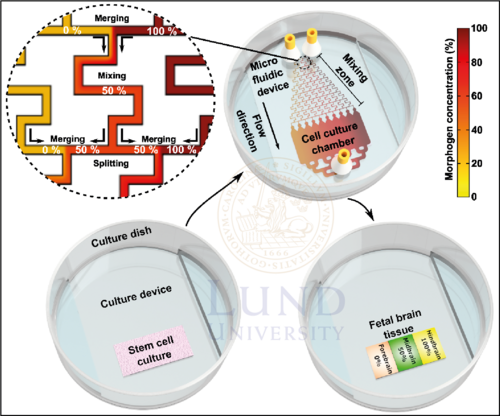
Figure 1. The MiSTR system. After hESCs are seeded on a matrigel, they are subsequently exposed to a gradient of GSK3i which generates neural progenitor cells of either forebrain, midbrain or hindbrain. The patterned tissue can then be dissected for bioassays such as qRT-PCR or single cell-RNA sequencing for characterization.
The MiSTR model is composed of a two-part microfluidic perfusion cell culture system: (1) a microfluidic PDMS device and (2) a bottom PDMS culture device. The bottom device contains a matrigel on which the hESCs are residing, while the microfluidic device contains the microfluidic gradient generator and the cell culture flow chamber. Alignment of the two devices creates a closed system, allowing for perfusion of culture media. To avoid the risk of leakages, a holder is used to tighten the microfluidic system. Culture media with no GSK3i (0%) and 2µM GSK3i (100%), are pumped into the microfluidic system through separate inlets. A gradient of GSK3i is then created by sequential merging-mixing-splitting of culture media fractions with different GSK3i concentrations in the gradient generator. As the media fractions with 10 discrete concentrations of GSK3i merge into the culture flow chamber, the discrete concentrations are allowed to mix into a continuous linear gradient.
In the culture chamber, hESCs are constantly exposed to the linear gradient for 9 days, stimulating them to differentiate into different neural progenitor cells depending on the local concentration. For another 5 days, the cell culture is simply provided culture media for proliferation. Once a patterned tissue is acquired, the microfluidic device is removed, and the tissue is dissected into 5 equal-sized parts for characterization with bioassays such as qRT-PCR or single cell-RNA sequencing.
References
[1] Nordstrom, U. et al. Progressive induction of caudal neural character by graded Wnt signaling, Nat Neurosci. 5, 525-532 (2002)
[2] Ribes, V. et al. Establishing and interpreting graded Sonic Hedgehog signaling during vertebrate neural tube patterning: the role of negative feedback. Cold Spring Harb. Perspective Biol. 1, a002014 (2009)
[3] Demers, C.J. et al. Development-on-chip: in vitro neural tube patterning with a microfluidic device. Development 143, 1884-1892 (2016)
