Biomechanics Masters thesis suggestions
Interested?
If you are interested in any of the projects below, please contact Hanna Isaksson, hanna.isaksson@bme.lth.se
Modeling Achilles tendon loading during gait
Objective: Investigate the magnitude of loading of the Achilles tendon during gait, by modeling the hindlimb during gait.
Approach: Physiological musculoskeletal modeling: Investigating physiological loading of the Achilles tendon fascicle using an OpenSim implementation of a Rat hindlimb / alt full rat. Investigate effects of Botox injection/muscle paralysis on the loading.
Background/Relevance/Application: The Achilles tendon transfers load between different calf muscles and the heel [1]. However, the magnitude of loading by the different calf muscles on the Achilles tendon is unknown.
Collaboration opportunities: Potential access to rat gait data [2] by Prof. M. Kersh, university of Illinois.
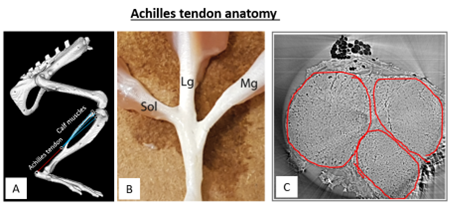
Figure. A) OpenSim implementation of a rat hindlimb to study muscle-tendon loading under physiological conditions B) Picture of a dissected rat Achilles tendon, displaying the separate fascicle-muscle connections C) CT image of a cross-section of a rat Achilles tendon to identify fascicles
Student background: Basic knowledge in finite computational modeling and mechanics.
Important literature:
[1] Lee AH, Elliott DM (2019) Comparative multi‐scale hierarchical structure of the tail, plantaris, and Achilles tendons in the rat. Journal of anatomy 234:252-262
[2] Song H, Polk JD, Kersh ME (2019) Rat bone properties and their relationship to gait during growth. Journal of experimental biology 222: jeb2035554
For more info, please contact: Biomechanics group, Professor Hanna Isaksson, hanna.isaksson@bme.lth.se
Towards dynamic modelling of a fall on the hip
Objective: Create a dynamic model of a human fall on the hip and obtain ground impact force and direction.
Approach: Adjust an open access model of the human body in OpenSim (https://simtk.org/) to replicate a fall on the hip. Read out the dynamic impact forces on the hip and use this data as input for a finite element model.
Background/Relevance/Application: In our group we have developed finite element models of the proximal femur (see figure). These models have been validated against mechanical experiments. The boundary conditions in the experiments were set to replicate a fall to the side. However, no 2 falls are exactly the same. People have varying anatomies, not only affecting the strength of the femur, but also the impact during a fall. To obtain a more accurate description of the forces acting on the hip an adjustable model of a human body falling is required
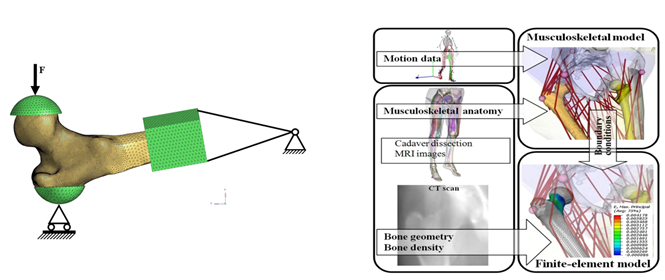
Figure. Left) Finite element model of a femur with boundary conditions replicating ex vivo mechanical testing Right) Image from: [1] showing the concept of including a finite element model in a full body musculoskeletal model.
Student background: Mathematics, basic knowledge in numerical analysis or finite element modeling, and matlab (or strong knowledge in another coding language).
Important literature: [1] Martelli et al. (2014) Strain energy in the femoral neck during exercise, Journal of Biomechanics
For more info, please contact: Biomechanics group, Professor Hanna Isaksson, hanna.isaksson@bme.lth.se
Image analysis of phase contrast enhanced synchrotron X-ray tomography of human meniscus
Objective: Characterize the microstructural organization of fibers in human medial menisci statically and when dynamically compressed. Determine the meniscus microstructural response to loading by tracking fiber rearrangements during in situ loading.
Background: Osteoarthritis (OA) is a "wear-and-tear" type of degenerative arthritis that mostly occurs in people above 50 years. Knee OA is associated with the degeneration of the meniscus. The meniscus, a crescent-shaped disc of fibrocartilage that is located between the surfaces of the femur and tibia in the medial and lateral compartments of the joint. It is composed of water and specifically hierarchically arranged collagen, which serves to distribute the load in the knee. Structural and compositional changes are known to occur in the meniscus due to OA, however, the details of how the degeneration evolves are still unknown. The wide aim of this study is to extend the current understanding of osteoarthritis by combining high resolution imaging with mechanical compression to visualize the altered response of the tissue due to degeneration at the micorscale.
Approach: The candidate will perform the analysis of static and dynamic X-ray phase contrast imaging datasets of human meniscus, acquired with the rheometer setup at the TOMCAT beamline (Paul Scherrer Institute). Focus on the analysis of fiber orientation, and how it changes along the in-situ stress-relaxation protocol applied. Possibility to correlate results to mechanical data and to perform digital image correlation to identify of micro deformations and strain distribution in the tissue.
![]()
Figure. A) Human meniscus and a 5mm plug used for imaging and in situ loading. B)setup for in situ loading at the TOMCAT beamline. C) Phase-contrast enhanced synchrotron X-ray volume showing the meniscus internal structure. D) Meniscus during compression. In plane fiber orientation and orientation distribution along the meniscus longitudinal plane.
Student background: knowledge in 3D image analysis and programing.
For more info, please contact: Biomechanics group, Professor Hanna Isaksson, hanna.isaksson@bme.lth.se
The effect of viscoelastic properties of ligaments on knee joint mechanics
Objective: Implementation of viscoelastic properties of ligaments into finite element models to estimate their effect on knee joint mechanics.
Background: Ligaments provide stability to the knee joint and play an important role in restraining motion. Knee ligaments have shown intrinsic viscoelastic properties which control the excessive motions in the joint. Simpler material representations have been implemented in 3D finite element knee joint models previously. However, ligament viscoelastic material models have not been studied in those models, and the potential effect of those properties on knee joint mechanics remains unknown.
Approach: The candidate will implement simpler material models as well as a viscoelastic material model for ligaments into a 3D finite element model using the software Abaqus. The kinematics and kinetics of the knee joint as well as cartilage stresses and strains will be evaluated for different ligament material models.
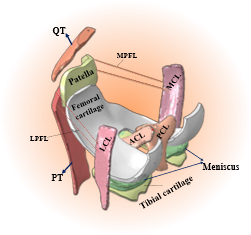
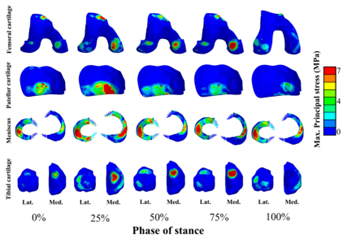
Figure. 3D finite element knee joint model with a solid representation of ligaments. Mechanical response of knee tissues can be estimated as a function of the stance phase of the gait cycle.
Student background: Basic knowledge in material physics.
Relevant literature:
[1] Orozco GA et al. (2018). The effect of constitutive representations and structural constituents of ligaments on knee joint mechanics. Scientific reports, (8), 2323.
[2] Räsänen L.P. et al. " Three-dimensional patient-specific collagen architecture modulates cartilage responses in the knee joint during gait." Computer Methods in Biomechanics and Biomedical Engineering 19 (2016): 1225-1240.
[3] Bonifasi-Lista. C., et. al. Viscoelastic properties of the human medial collateral ligament under longitudinal, transverse and shear loading. Journal of Orthopaedic Research, 23, (2005). 67-76.
For more info, please contact: Biomechanics group, Professor Hanna Isaksson, hanna.isaksson@bme.lth.se
Nanoscale characterization of collagen structural response to in situ loading in Achilles tendons
Objective: Quantitatively explore the axial and lateral collagen fibril responses to in situ loading in intact Achilles tendons, and their link to overall tissue behavior.
Background/Relevance: Tendons are responsible for transducing loads from muscles to bone, allowing energy efficient movement. They can withstand high tensile loads and have complex load-dependent biomechanical properties. Their mechanical properties are linked to their hierarchical structure, with collagen as their main load bearing unit. At the nanoscale, collagen molecules periodically arrange into fibrils, which can be studied using X-ray diffraction. The mechanical responses of the nanoscale collagen fibrils and their relation to the overall tissue behavior is not yet fully understood.
Approach: In situ loading combined with small- and wide-angle X-ray scattering (SAXS/WAXS) will be conducted on intact rat Achilles tendons at coSAXS, MAX IV. From this data, both axial and lateral fibril mechanical response as well as changes in intrafibrillar hydration during loading will be assessed. Additionally, different load rates will be used to evaluate the strain rate dependency of the fibrils. The candidate will participate in preparations before and during the beamtime. After the experiment, the candidate will use existing implementations as well as explore, develop and implement new analysis of the SAXS and WAXS data to quantify fibril strains, changes in hydration, strain rate dependency and the global tissue scale mechanical responses.
Collaboration opportunities: The work is part of a collaborative project between Lund University and MAX IV.
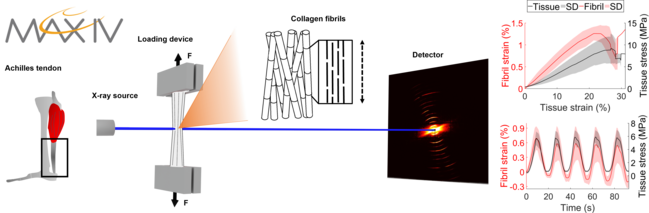
Figure. Achilles tendons will be mounted in a tensile device at the coSAXS beamline, MAX IV and loaded in different loading scenarios. Simultaneously, SAXS and WAXS patterns will be recorded to study the fibril behavior. The fibril and tissue mechanical responses will be compared.
Student background: Basic knowledge scattering and diffraction, experimental mechanics and MATLAB.
For more info, please contact: Biomechanics group, Professor Hanna Isaksson, hanna.isaksson@bme.lth.se
High definition 3D tracking of tendon collagen fibers
Objective: Develop a computer algorithm to 3D track fibers inside the complex Achilles tendon tissue. Determine how fiber sizes change in different regions of the tendon and under different loading conditions. Determine the (dis)continuity of fibers (which is currently under debate).
Background: Tendons are soft tissues composed of hierarchically arranged collagen fibers (30%) and water (70%). Achilles tendons are mechanoresponsive, i.e. they actively adapt to their in vivo mechanical loading environment over time. Using high-resolution tomography and automized 3D computer analysis this experiment aims to investigate how the microstructure is affected by altered in vivo mechanical loading/unloading. To date the full relationships between structure and function throughout the tendon hierarchy is yet to be elucidated. Understanding how loading influence the tendon tissue is essential to preserve a correct mechanical function of tendons which, in turns, affect the overall skeletal health. The data that will be analyzed in this project are high resolution volumesacquired by phase-contrast enhanced synchrotron X-ray tomography. To our knowledge this cutting-edge technique was never use before to study Achilles tendon structure before.
Approach: The candidate will develop and validate an algorithm to segment single fibers, calculate fiber sizes and fiber geometries, and follow fiber trajectories three-dimensionally.
![]()
Figure: A) schematic representation of an Achilles tendon. B) Phase-contrast enhanced synchrotron X-ray volume showing the tendon internal structure. C) volume rendering showing some collagen fibers.
Student background: knowledge in 3D image analysis and programing.
For more info, please contact: Biomechanics group, Professor Hanna Isaksson, hanna.isaksson@bme.lth.se
Development of a dynamic FE model of a fall to the side
Objective: Develop a computational 3D finite element (FE) model of the hip impact during a fall to the side. The goal is to predict whether the fall would result in a hip fracture or not.
Background: Hip fractures are a very common issue among elderly people and an ever-increasing socio-economic issue in an ageing population. Computational 3D FE models can help to predict bone strength and consequently the risk of fracture. However, most current FE models simulate quasi-static loads on the bones, whilst over 90% of hip fractures result from a sideways fall, which is a clear dynamic event.
Approach: The candidate will develop and validate a computational 3D FE model using the determined global and local strains, trabecular bone microstructures, and mineral density distributions from µCT images that have been acquired while bone deforms in situ under compressive loading.
Application: An existing subject-specific FE modeling pipeline of human femurs that was developed in our group will be used as the starting point. This pipeline includes strain-rate dependent material properties with specific strain limit values for yield and failure. First, this material model will be adjusted to work in dynamic models that are solved using explicit solvers. The second part of the project consists in modeling the soft tissue around the hip using CT scans and including that in the FE model to evaluate the damping effect of soft tissue on the fracture outcome.
Figure. The validated quasi-static FE models in developed at the biomechanics group at Lund University. This project will adjust the constitutive relations to work in case of a dynamic fall, where an impact speed to the ground is imposed, and add soft tissue padding obtained from CT scans to the model.
Student background: Knowledge of finite element modeling (FHL066 or analogous course is highly valued), image analysis and solid mechanics.
For more info, please contact: Biomechanics group, Professor Hanna Isaksson, hanna.isaksson@bme.lth.se
A computational model of the human Achilles tendon
Objective: Characterize the 3D Achilles tendon behavior by establishing a realistic human Achilles tendon model by describing realistic loading, geometry, material properties.
Approach:
- Literature exploration: Geometrical characterization of the tendon fascicles and establish appropriate boundary conditions for the separate subunits of the Achilles tendon.
- Experimental mechanical analysis: Tensile mechanical testing of the different tendon fascicles
- Finite element analysis: Combining acquired information to build a fascicle-level Achilles tendon model of the human tendon in a finite element framework
Background: The Achilles tendon transfers load between different calf muscles and the heel bone. This anatomy induces large inhomogeneities in external loading and internal properties. However, deep understanding of these inhomogeneities is lacking, and the tendons is mostly considered one cylindrical homogenous structure.
Collaboration opportunities: Potential collaboration with the biobank for obtaining biological samples for experimental characterization of geometrical properties and boundary conditions, or with Linköping University to utilize clinical CT images of the human lower limb.
Figure. A) Leg anatomy B) identifying the 3 subunits of the Achilles tendon and C) characterizing these dimensions and their D) orientations*. E) Comparison of the old simplified tendon geometry and a prototype for a more realistic geometrical model.
Student background: Basic knowledge in solid mechanics and experimental mechanics or finite element modeling, depending on focus above. Interest in biomechanics and image analysis is highly beneficial.
Important literature:
*Edama et al. (2015) The twisted structure of the human Achilles tendon. Scand J Med Sci Sports 25: e497-e503
For more info, please contact: Biomechanics group, Professor Hanna Isaksson, hanna.isaksson@bme.lth.se
Computational model on bone fracture mechanisms
Objective: Develop and validate a computational 3D finite element (FE) model on bone fracture mechanisms for improved predictions of fractures.
Approach: The candidate will develop and validate a computational 3D FE model using the determined global and local strains, trabecular bone microstructures, and mineral density distributions from µCT images that have been acquired while bone deforms under compressive loading.
Application: Trabecular bone tissue from cadavers with normal and low density bone is used. Using combined micro tomographic imaging, in situ mechanical loading, and digital volume correlation (DVC), the global mechanical characteristics and local tissue strains of human trabecular bone have been assessed. After development and validation of the FE model, the roles of local tissue mineral density and trabecular bone structure to the local and global fracture resistance in trabecular bone are evaluated. The overall aim is to predict the mechanical integrity of trabecular bone tissue and bone material for a better understanding of the damage and fracture mechanisms.
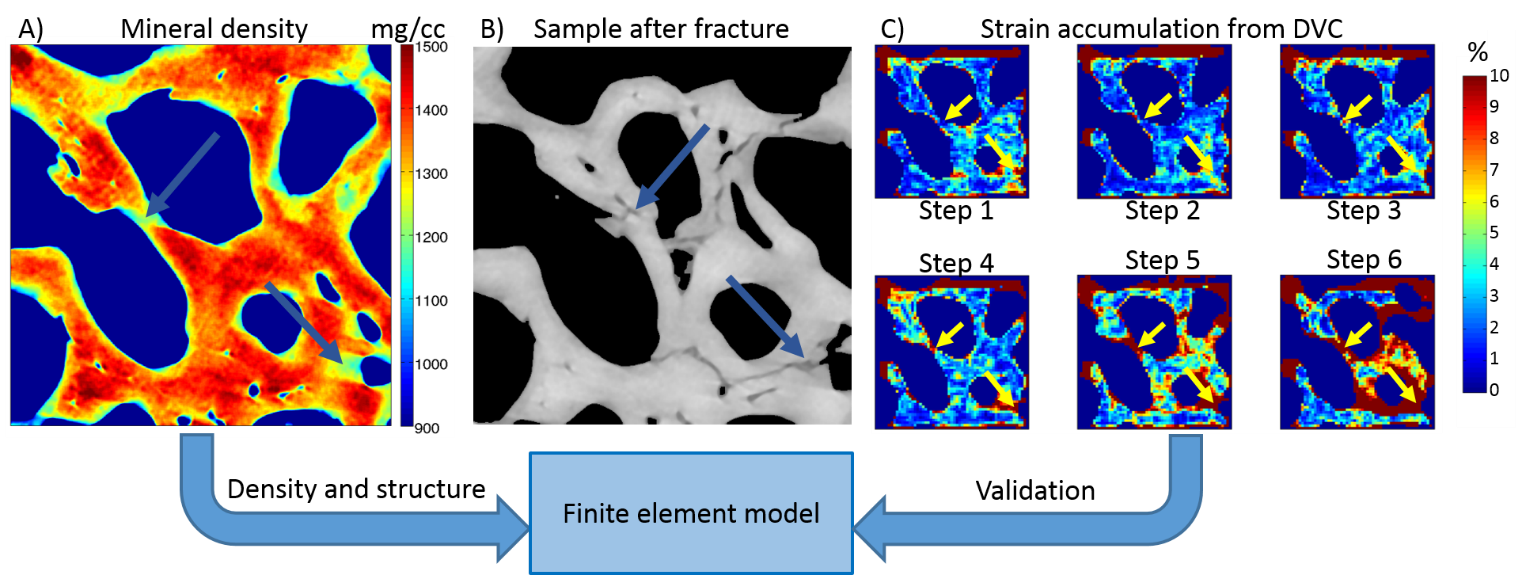
Collaboration opportunities: The work is part of a collaborative project between Lund University (Lund, Sweden, Department of Biomedical Engineering), and University of Eastern Finland (Kuopio, Finland, Department of Applied Physics). The thesis supervisors will be Mikael Turunen, Ph.D., (Kuopio) and Associate professor Hanna Isaksson, Ph.D., (Lund). The work will be performed mainly at University of Lund.
Student background: Basic knowledge in finite element modeling, image analysis and solid mechanics.
For more info, please contact: Biomechanics group, Professor Hanna Isaksson, hanna.isaksson@bme.lth.se
