WBC
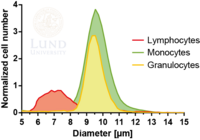
Subpopulations of white blood cells
White blood cells (WBC), leukocytes, play an important role in the human immune system and can be divided into three main subpopulations: lymphocytes, monocytes, and granulocytes. Immediate and individual access to these WBC subtypes holds significant value in research as well as clinical applications. Simultaneous fractionation of the three subsets poses a great challenge as they are somewhat similar in size and have a size overlap as can be seen in the figure to the right.
White blood cell separation
Petersson et al. (2007) pioneered multi-outlet acoustophoretic fractionation separating RBCs, WBCs and platelets concurrently. The purities were not great however, partly because of size overlap, but also because the migration of the cells not only was due to the acoustic response but due to the position of the cells in the flow channel. Grenvall et al. (2015) introduced an acoustic pre-alignment step before the multi-outlet separation in which all cells were focused in the same flow vector, effectively eliminating the hydrodynamics effects and allowing the separation to be purely on the acoustophysical properties of the sample. The performance of the acoustophoretic separation showed high purities of lymphocytes and granulocytes.
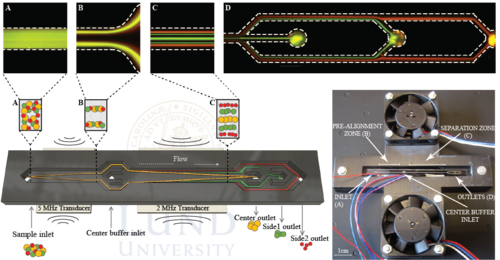
In order to achieve higher throughput Urbansky et al. (2019) further developed the concept of Grenvall and Petersson. Using and elongated chip with pre-alignment as well as a novel chip holder, the WBC fractionation performance could be increased 35 compared to previous work. The chip holder comprised two fans that enabled air-cooling of the chip effectively transporting away excess heat which may arise at high operating voltages that is needed during high throughput separations. The chip holder also introduced minimal contact points with the chip in order to preserve as much acoustic energy as possible. Illustration of the holder and chip can be seen below.
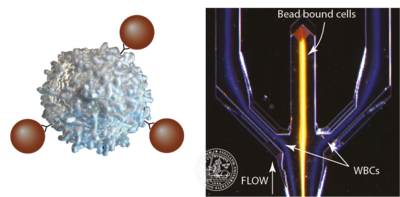
Affinity beads with antibodies
When the acoustophysical properties of cells becomes too similar, separation will be very challenging. One way of dealing with these limitations is to use affinity beads with specific antibodies that will only couple to the cell that has the specific surface marker. The cell/bead complex will then differ in size and in acoustic impedance from the non targeted cells which will allow for an acoustic separation of the targeted cell type. This has been used to separate CD4+ T-cells from a WBC sample (Lenshof, 2014) as well as CD8+ T-cells (Urbansky 2016).
References
Petersson F., Åberg L., Swärd-Nilsson A-M. and T. Laurell, Free flow acoustophoresis: microfluidic-based mode of particle and cell separation, Analytical Chemistry, 2007, 79, 5117-5123
Grenvall C. Magnusson C., Lilja H. and T. Laurell, Concurrent isolation of lymphocytes and granulocytes using prefocused free flow acoustophoresis, Analytical Chemistry, 2015, 87, 5596-5604
Urbansky A., Olm F., Scheding S., Laurell T. and A. Lenshof, Label-free separation of leukocyte subpopulations using high throughput multiplex acoustophoresis, Lab on a chip, 2019, 19, 1406-1416
Lenshof A., Jamal A., Dykes J., Urbansky A., Åstrand-Grundström I., Laurell T and S. Scheding, Efficient purification of CD4+ lymphocytes from peripheral blood progenitor cell products using affinity bead acoustophoresis, Cytometry A, 2014, 85, 933-941
Urbansky A., Lenshof A., Dykes J., Laurell T. and S. Scheding, Affinity Bead Mediated Enrichment of CD8+ Lymphocytes from Peripheral Blood Progenitor Cell Products using Acoustophoresis, Micromachines, 2016, 7, 101
